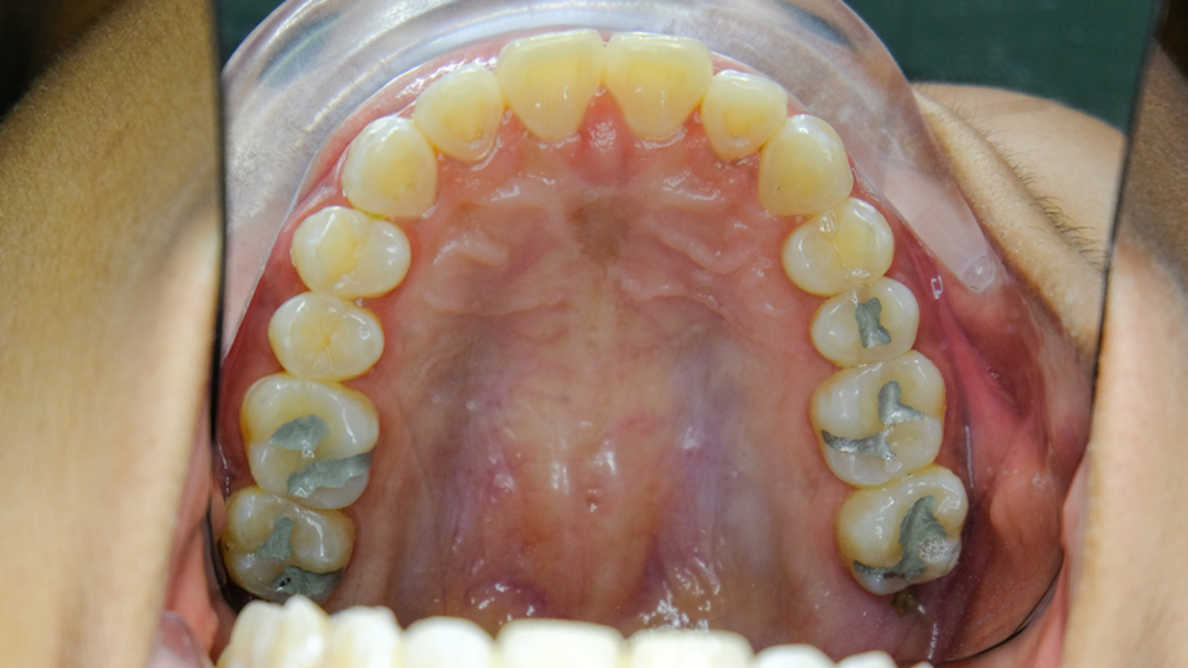
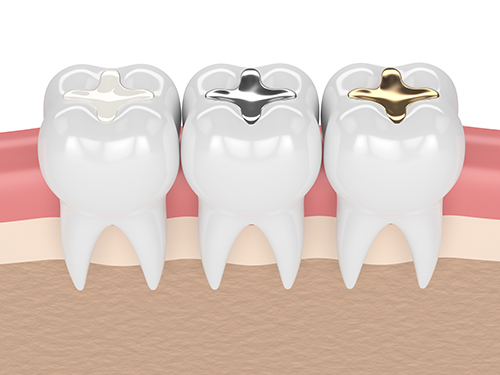
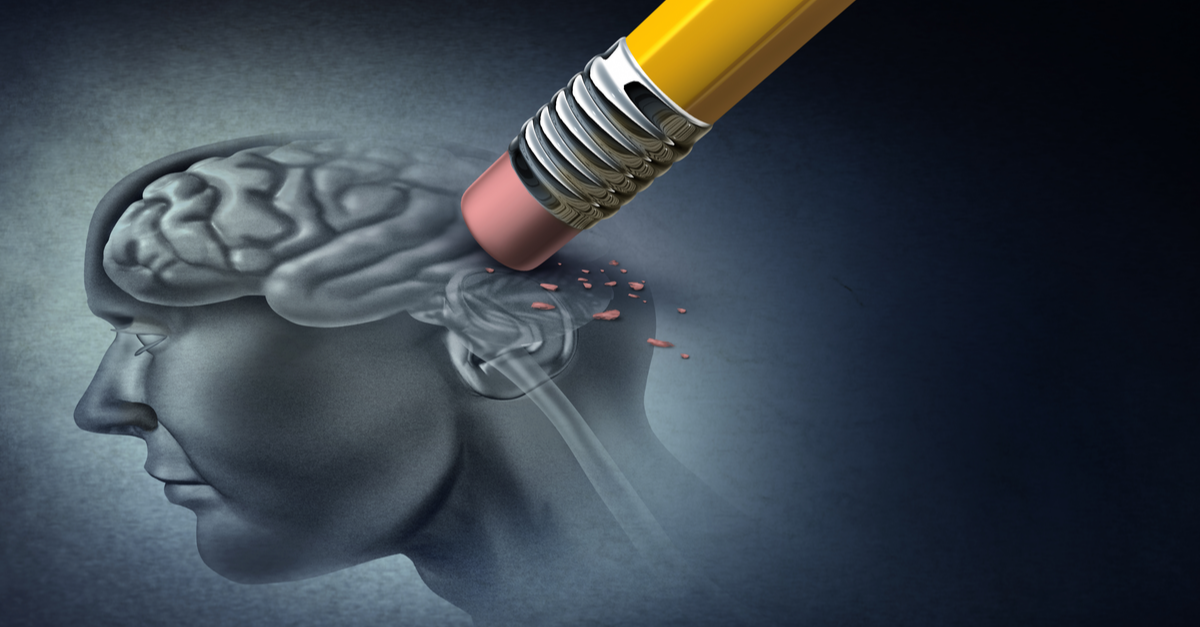
Recommendations About the Use of Dental Amalgam in Certain High-Risk Populations: FDA Safety Communication
Date Issued: September 24, 2020
The U.S. Food and Drug Administration (FDA) is providing recommendations about the use of dental amalgam in certain groups of people who may be at greater risk to the potential adverse health effects of mercury exposure, to include:
- Pregnant women and their developing fetuses;
- Women who are planning to become pregnant;
- Nursing women and their newborns and infants;
- Children, especially those younger than six years of age;
- People with pre-existing neurological disease;
- People with impaired kidney function; and
- People with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.
For over 20 years, the FDA has been reviewing, considering and holding public discussions regarding the scientific literature and other evidence on the safety of dental amalgam. Key among our findings are the uncertainties about the acceptable reference exposure levels for mercury vapor (gas), the potential for mercury to convert to other mercury compounds in the body, and whether the degree of accumulation of mercury from dental amalgam results in negative (adverse) health outcomes. The FDA held a meeting of our Dental Products Panel of the Medical Devices Advisory Committee in December 2010External Link Disclaimer and a meeting of our Immunology Devices Panel in November 2019 to discuss these uncertainties. Elemental mercury used in dental amalgam is known to cause adverse health effects, particularly when the extent of exposure is high, in individuals who have reduced ability to remove mercury from their bodies, and in individuals who are sensitive to mercury. Although the majority of evidence suggests exposure to mercury from dental amalgam does not lead to negative health effects in the general population, little to no information is known about the effect this exposure may have on members of the specific groups listed above who may be at greater risk to potential negative health effects of mercury exposure. Accordingly, the FDA recommends that non-mercury restorations (fillings) such as composite resins and glass ionomer cements be used, when possible and appropriate, in people who may be at higher risk for adverse health effects from mercury exposure.
We welcome you to read the full paper HERE!